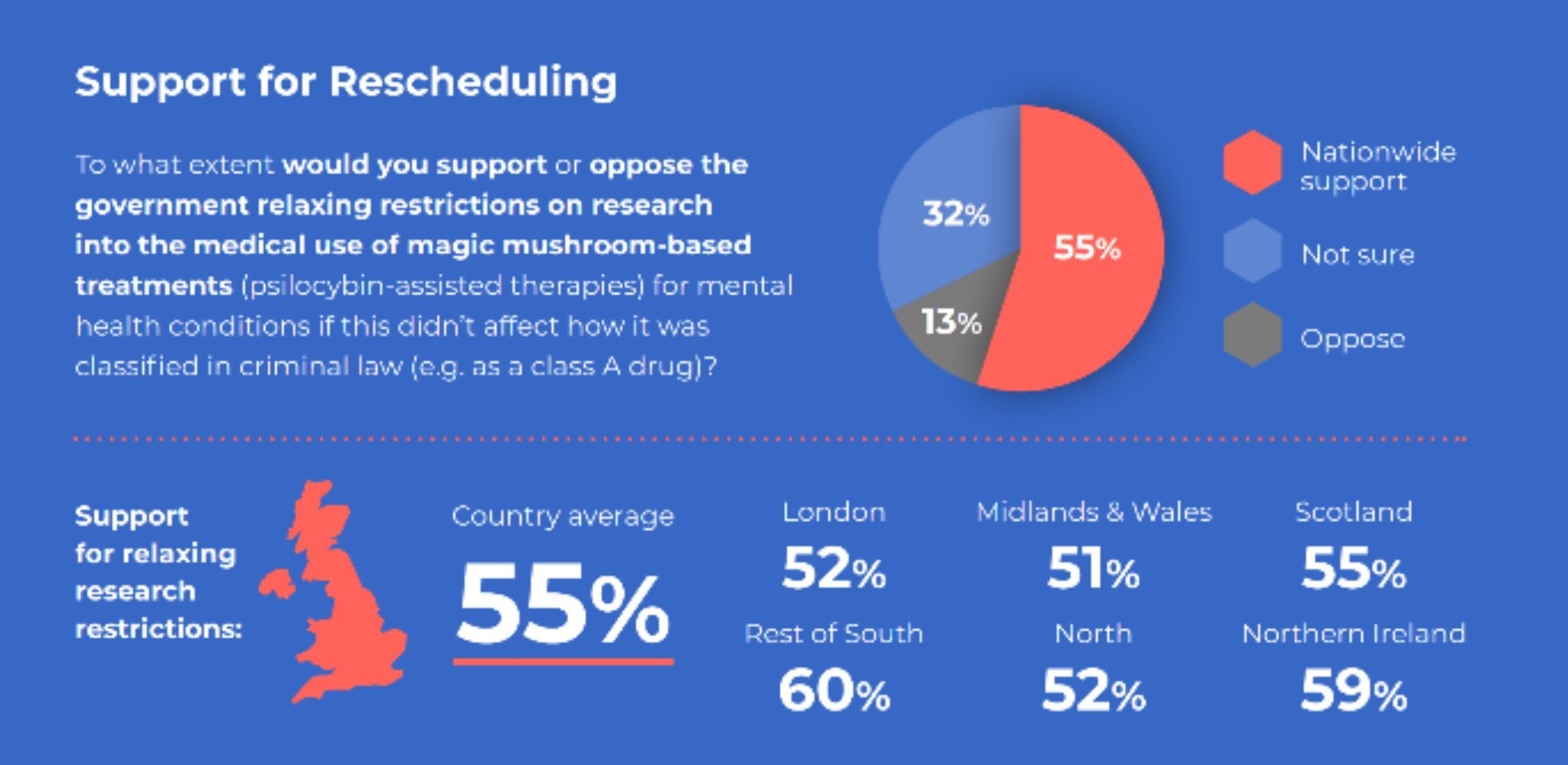
Recent YouGov poll data shows that across all demographics a majority (55%) of the UK public supports a change in the law to facilitate research into psilocybin for treatment development, with the don’t knows excluded, this is a 4-1 view.
In line with this majority view, the chairman of the CDPRG, Crispin Blunt MP, has revealed that in an office meeting on 26th of May 2021 the Prime Minister confirmed that he has signed approval for the rescheduling of psilocybin and other psychedelics in a move that would unlock the science that could save millions of lives - yet months later the UK Home Office (HO) still fails to act.
Psilocybin has potential as a treatment for a plethora of psychiatric and medical conditions like PTSD1, OCD2 and Depression3, but many possible trials, which could address a huge unmet clinical need both in the UK, and globally, fall by the wayside. This is due to the extended time frames, increased costs, and prohibitive stigma associated with researching controlled drugs in Schedule 1 of the Misuse of Drugs Regulations 2001 (MDR 2001).
Neuroimaging research undertaken by Dr Robin Carhart-Harris and Professor David Nutt at Imperial College London in 2012, found that psilocybin affects areas of the brain that are implicated in depression, making the chance discovery that it could be used as a treatment for the condition. But to undertake this research multiple Schedule 1 licenses had to be obtained from the HO, taking several years and costing upwards of £5000 per license. The study was the first to research the brain on psilocybin and was conducted with 30 healthy volunteers, with the cost per dose estimated at £1500 - ten times higher than if the substance was not in Schedule 1.
Even though psilocybin has been recognised by the HO as a promising medical and psychiatric intervention, in practice the HO fails to action the simple repeated request to commission the Advisory Council on the Misuse of Drugs (ACMD) to review its designation as a Schedule 1 substance and act on its recommendations. The reason given for not actioning the review of psilocybin’s scheduling is that it has not yet achieved market authorisation. But market authorisation is not a prerequisite for rescheduling, and as such the decision not to call for an urgent ACMD review of psilocybin on this ground is unfounded.
The market authorisation and the scheduling of a particular substance are related, but not interdependent upon one another. The Misuse of Drugs Act (MDA 1971) was intended to be flexible and forward looking, and its associated MDR 2001, are intended to be permissive — facilitating policy changes in line with the needs of society and the emerging evidence.
Market authorisation as a prerequisite to any such permission is nowhere mentioned. Thus, it is manifestly possible for the Home Secretary to request an ACMD review of the scheduling of psilocybin, if minded that it was right to do so, prior to it achieving market authorisation. Though the ACMD can commission its own work, it lacks the capacity to do so, therefore a review of the psilocybin scheduling must come in practice from the HO.
The route to rescheduling via market authorisation i.e., the route popularly misunderstood to be the only one available, does not consider the utility of certain substances in scientific research. Its value being solely in its capacity to facilitate experimental scientific research; never reaching market authorisation. This situation is analogous to that of psilocybin and other Schedule 1 psychedelics such as LSD, DMT, MDMA, Bufotenin, Mescaline, the 2Cs and others of which we are yet to discover the potential.
Disproving beyond further discussion that market authorisation is not a necessary precursor to rescheduling, is the case of cannabis-based products for medicinal use in humans (CBPM) which were rescheduled by statutory instrument in 2018, almost 20 years after a House of Lords committee recommended moving cannabis to Schedule 2. It is important to note that there is already substantially more evidence for the safety and efficacy of psilocybin than there was, and still is, for medical cannabis.
Following the precedent that this has set for the rescheduling of controlled drugs from Schedule 1 to Schedule 2 prior to market authorisation as a medicinal product, we propose a similar wording for the case of psilocybin — with an additional provision precluding the prescription of unlicensed medicinal products outside of clinical and experimental research.
More information on these issues can be found in our report Medicinal Use of Psilocybin and the briefing paper Misinterpretation of the Misuse of Drugs Act 1971 and the Misuse of Drugs Regulations 2001 in the case of the call for the rescheduling of psilocybin.
Words: Timmy Davis (Psilocybin Rescheduling Project Manager) and Iram Salam (Junior Researcher)